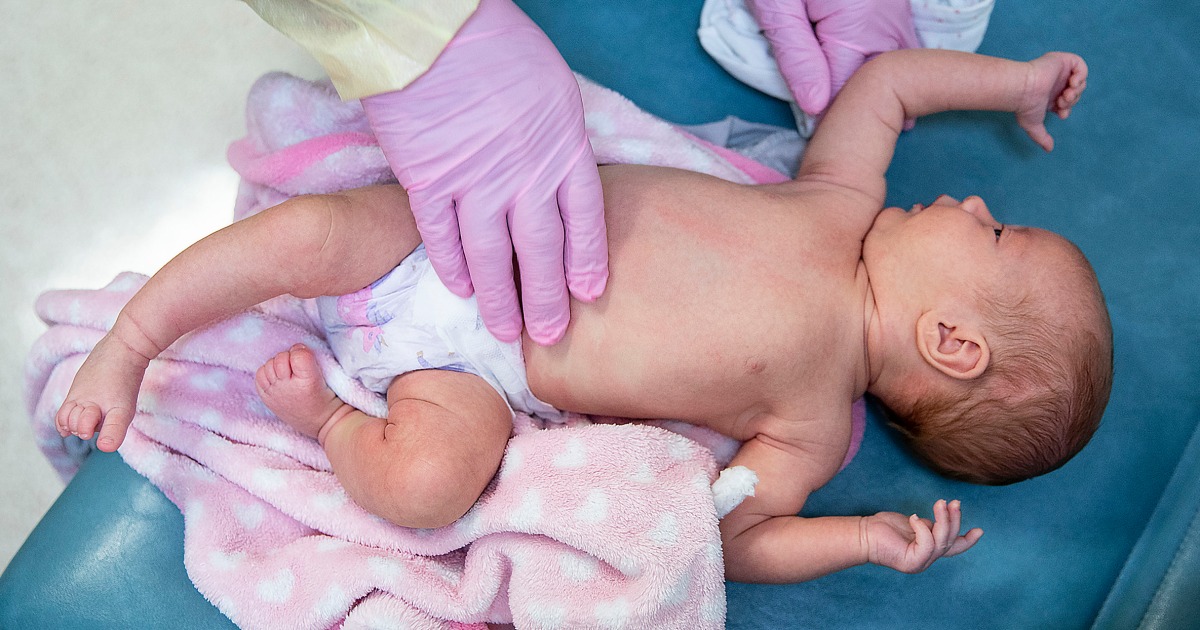
An independent advisory committee to the Food and Drug Administration will decide Thursday whether to recommend an RSV vaccine for infants.
If it is eventually approved, the shot, which is made by Pfizer and administered to pregnant mothers, would be the first RSV vaccine for infants in the U.S.
“Before the pandemic, RSV was the No. 1 cause of infant hospitalization in the United States, so this is a big deal,” said Dr. Ofer Levy, the director of the Precision Vaccines Program at Boston Children’s Hospital. Levy is a temporary voting member of the FDA panel but isn’t involved in Thursday’s vote.
If the committee votes in favor of the vaccine, the FDA must still approve it — a process that could take months — but it is likely to follow the advisers’ recommendation.
The agency approved the world’s first RSV vaccine this month, made by pharmaceutical giant GSK, but the shot is only for adults ages 60 and up.
Pfizer also has an RSV shot for older adults up for FDA approval this month. The FDA advisory panel in late February recommended the shot for people ages 60 and up. It’s the same vaccine that could be given to pregnant people.
The shot the FDA committee is evaluating Thursday would be given to pregnant people at 24 to 36 weeks’ gestation. The protective antibodies transfer to infants through the placenta.
In a clinical trial with nearly 7,400 participants, the vaccine lowered the risk of severe disease from RSV among infants by 82% within roughly three months after birth. By around six months, efficacy was around 69%. Infants 6 months and younger are especially vulnerable to severe cases of RSV, which stands for respiratory syncytial virus.
The shot also lowered the risk of developing respiratory disease from RSV that required doctors’ visits by 51% within about six months. After that, however, the vaccine didn’t appear to make a big difference.
“Maternal immunization looks like an important piece of the puzzle, but we’re going to need more to shield into the second half of the first year and beyond,” Levy said.
The most common side effects of the shot reported among pregnant women were fatigue, headache, muscle pain and injection site pain.
In a briefing document released this week, the FDA said safety data from the trial seemed “generally favorable.”
However, the agency noted that there was a slightly higher rate of preterm births — defined as before 37 weeks’ gestation — among people who received the vaccine (5.7%) versus those who got a placebo (4.7%). The difference wasn’t statistically significant, however, so it’s unclear whether it was vaccine-related.
Both rates were lower than the rate of preterm births in the general population: around 10%, according to the Centers for Disease Control and Prevention.
Last year, the pharmaceutical giant GSK halted its trial of an RSV vaccine for infants after it showed a higher preterm birth rate among some vaccine recipients.
Levy said researchers generally pay close attention to any potential risk of preterm births among vaccines given to pregnant people.
“There’s always the background concern: Are you inducing some inflammation that could be a problem? Because the body reads inflammation as ‘the woman’s no longer safe, let’s get the baby out.’ So you want a fairly bland vaccine,” he said.
In healthy adults, lower respiratory illness caused by RSV is typically mild, but it can be more severe in babies and older adults. The virus kills up to 300 children under 5 every year in the U.S. and up to 10,000 people ages 65 or older, according to the CDC. Severe infections can result in pneumonia or bronchiolitis, which inflames airways and clogs them with mucus.
Eleven RSV vaccines (including GSK’s approved shot) are being actively studied in U.S. trials, according to data provided to NBC News by PATH, a nonprofit global health organization. Six are for older adults, and five are designed to protect infants or children.
A monoclonal antibody injection, which is given directly to infants and functions similarly to a vaccine, has already been approved in Canada, Europe and the United Kingdom. The FDA began reviewing data on that shot in January and is expected to decide this summer or fall whether to approve it.







Recent Comments