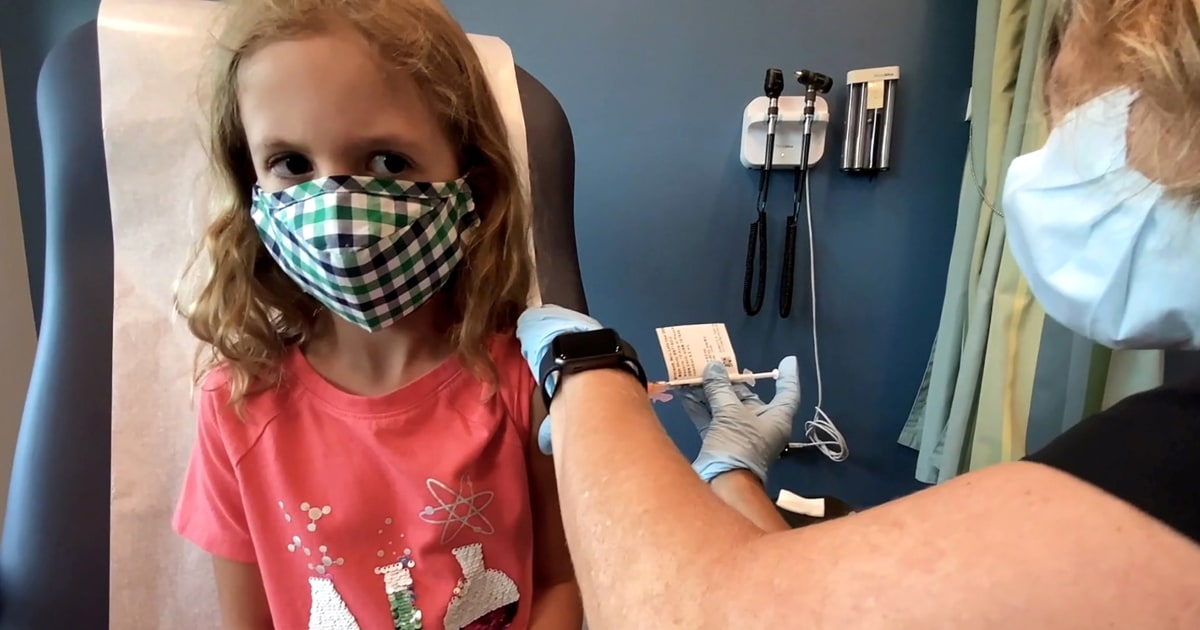
Pfizer could have data on how well its Covid-19 vaccine works in kids under age 5 by the end of the year, according to CEO Albert Bourla.
“We have a study in children 6 months to 2 years and then another cohort of children 2 to 5 years,” Bourla told NBC News chief White House correspondent Kristen Welker. “So [by] end of the year, beginning of next year, when we see the data, we will know more.”
As the omicron variant begins to spread globally, there are concerns about risks to young children. At a briefing Friday in South Africa, where the virus was first identified and infections have been surging, a top scientist said medical staff had begun to see an increase in admissions for kids under age 4.
Full coverage of the Covid-19 pandemic
Moderna CEO Stéphane Bancel, who joined Bourla in a joint interview at the Edward M. Kennedy Institute for the U.S. Senate at the University of Massachusetts Boston campus, also said his company is testing its vaccine in children, though he didn’t say when data could be available.
Last month, Moderna announced U.S. regulators were delaying their decision on the company’s vaccine for 12- to 17-year-olds while they study the rare risk of heart inflammation, known as myocarditis, which has been seen in a small number of young men who received the shot.
“With children, you want to go very slowly down in age … and start at a low dose and then slowly increase the dose level to find the right one,” he said. “So it’s taking a bit more time because of the safety of those children in those studies is very important to all of us.”
The pharmaceutical executives’ comments come as the Biden administration is urging all eligible Americans to get a booster shot of a Covid-19 vaccine over concerns about the omicron variant and the continued spread of the highly transmissible delta variant.
The omicron variant, in particular, has mutations that suggest it could evade the protection provided by vaccination or natural infection.
Download the NBC News app for full coverage of the Covid-19 pandemic
Pfizer’s vaccine has been cleared for people as young as 5, while Moderna’s vaccine has been authorized for adults. The only group in the U.S. not eligible to get a shot are children under age 5.
In the interview with NBC News, Bancel urged the public to stay “calm” as federal health officials and drugmakers await data on how well the existing Covid-19 shots work against the new variant.
“We are looking at all the data because it’s part of the dataset that is building slowly but will be much smarter in a few weeks about how this virus behaves,” he said.
Follow NBC HEALTH on Twitter & Facebook.







Recent Comments