But growing usable human tendon cells—which need to stretch and twist—has proved trickier. Over the past two decades, scientists have encouraged engineered tendon cells and tissue to grow and mature by repeatedly stretching them in one direction. However, this approach has so far failed to produce fully functional tissue grafts that could be used clinically, in human bodies.
A new study, published in Nature Communications Engineering today, shows how humanoid robots could be used to make engineered tendon tissue that is more like the real thing.
“The clinical need is clearly there,” says Pierre-Alexis Mouthuy from the University of Oxford, who led the team. “If we can create grafts in vitro that can be of good enough quality to use in clinics, that would be really helpful for improving outcomes in patients. Any improvement would be more than welcome.”
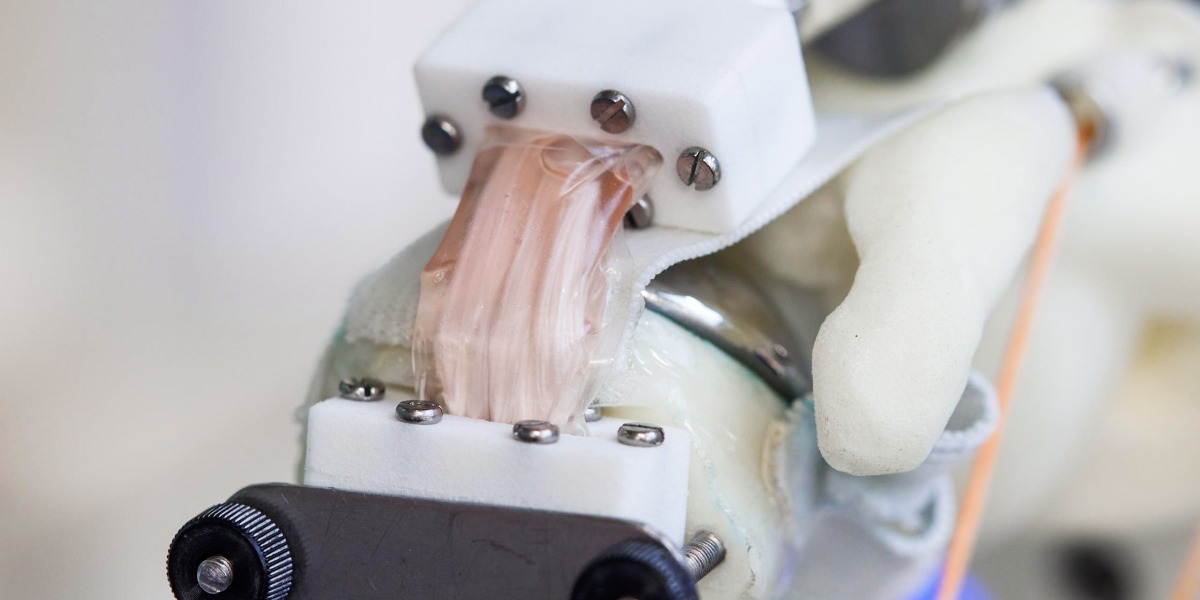
The first step involved redesigning the test chamber that houses the cells, known as a bioreactor, to attach it to a humanoid robot shoulder that can bend, push, pull, and twist cells in the same way musculoskeletal tissues would.
While traditional bioreactors resemble rigid boxes, the team created a flexible one in which human fibroblast cells—elongated cells found in connective tissues—are grown on a soft plastic scaffold suspended between two rigid blocks. They attached this chamber to the robotic shoulder, which spent half an hour a day over 14 days replicating the kinds of raises and rotation movements a human would make.
Afterwards, the cells in the bioreactor were found to have reproduced more rapidly than samples that had not been stretched, and they expressed genes differently—although the researchers don’t know yet how that would translate to the quality of the graft. The team plans to investigate how cells grown in their new bioreactor compare with those grown in traditional stretch bioreactors.





Recent Comments